Introduction: Systemic amyloidoses are progressive, life-threatening diseases characterized by the deposition of amyloid protein fibrils of varying origin in different tissues/organ systems (Merlini G, et al. N Engl J Med 2003;349:583-96). The precursor amyloidogenic protein influences the disease's clinical course, and identification of the specific protein is essential because treatment varies substantially by subtype. While there are 36 known proteins that can aggregate as amyloid in humans (Benson MD, et al. Amyloid 2018;25:215-9), the two most prevalent protein subtypes causing cardiac amyloidosis are derived from immunoglobulin light chains (AL) and transthyretin (ATTR) (Kittleson MM, et al. Circulation 2020;141:e7-22). Both AL and ATTR subtypes often infiltrate the heart, resulting in a restrictive cardiomyopathy along with other multiorgan involvement. Appropriate classification, early identification, and prompt treatment may substantially improve clinical outcomes. Because AL disease occurs in the context of plasma cell dyscrasia, hematologists can play an important role in amyloidosis suspicion, diagnostic workup, and management. However, differentiation of AL and ATTR amyloidoses in patients with signs of cardiac dysfunction is often challenging, and a multidisciplinary approach, including referral to cardiologists, is recommended early in the patient diagnostic workup. To gain insights into hematologists' disease awareness and practices, we interviewed hematologists involved in amyloidosis patient care across the US.
Methods: A qualitative double-blind telephone survey was conducted between November 2019 and February 2020 of US hematologists who had diagnosed and/or treated at least 2 patients with AL amyloidosis over the past 2 years. The participants differed based on their experience in various clinical practice settings, including community hospital and private practices, academic institutions, and amyloidosis centers.
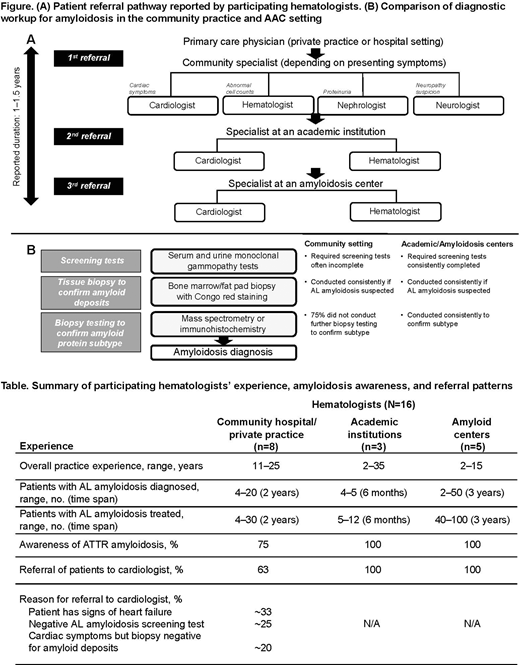
Results: A total of 16 hematologists participated in the survey (community hospital, n=3; community private practice, n=5; academic institution, n=3; amyloidosis center, n=5). Hematologists at amyloidosis/academic centers (AAC) reported that the AL amyloidosis patient's journey typically involved visits to multiple primary care physicians and specialists in the community over a prolonged period (approximately 1 to 1.5 years) before the patient received a diagnosis (Figure [A]). Community specialists' referrals to academic physicians within the same specialty, due to lack of familiarity with amyloidosis centers, contributed in part to the delay. Several differences were found between hematologists in the community and those at AAC in level of disease awareness and referral/testing practices (Table; Figure [B]). Hematologists in community practice were less likely to be aware of ATTR amyloidosis, refer patients with suspected amyloid to cardiologists, or conduct recommended screening/diagnostic testing. In contrast, hematologists at AAC were highly aware of ATTR amyloidosis, collaborated closely with cardiologists, and used recommended amyloidosis tests. Across practice settings, hematologists consistently conducted biopsies of bone marrow and fat pad in patients with suspected AL amyloidosis to confirm the presence of amyloid. After amyloid was confirmed with Congo red staining, 75% of community hematologists discontinued testing, without establishing the amyloid protein subtype; hematologists at AAC consistently assessed amyloid protein subtype using immunohistochemistry and/or mass spectrometry to differentiate AL and ATTR prior to initiating treatment. Diagnostic algorithms supporting AL and ATTR differentiation were consistently in place and followed at AAC but not in community-based practices.
Conclusions: Disease awareness, referral practices, and screening/testing procedures can differ between hematologists in the community setting and those in AAC. Community hematologists may benefit from additional education and wider use of diagnostic algorithms on AL/ATTR amyloidosis. Reinforcing the importance of cardiology referral and guidance on best practices for screening/biopsies/subtyping in patients with suspected amyloidosis who have cardiac symptoms should be prioritized.
D'Souza:Amgen, Merck, TenoBio: Research Funding; Akcea, Imbrium, Janssen, Pfizer: Consultancy. Costa Chase:Celgene: Speakers Bureau. Borham:Pfizer: Current Employment, Current equity holder in publicly-traded company. Bruno:Pfizer: Current Employment, Current equity holder in publicly-traded company.
Author notes
Asterisk with author names denotes non-ASH members.